ACE2: Receptor for COVID-19 Invasion
Feb 28, 2020

The recent outbreak of COVID-19 that started in Wuhan, China, has spread globally and caused major loss in human lives. The latest research has reported that ACE2 (angiotensin converting enzyme 2) is a functional receptor for COVID-19 to latch on and enter.View All COVID19 Products
The coronavirus enters human cell through the interaction of virus Spike protein (S protein) and ACE2 receptor on the cell membrane.
What is ACE2?
ACE2 is a carboxypeptidase that shares significant homologue with ACE. Human ACE2 is a type I integral membrane protein made of 805 amino acids (AA). It contains a 17 AA N-terminal signal area, a 22 AA hydrophobic transmembrane near the C-terminus and a 43 AA cytoplasmic domain. It is expressed in spleen, liver, retina, placenta, brain tissue, heart, coronary arteries, arteries, veins, endothelial tissue, macrophages, gastrointestinal system, lung alveolar epithelial cells and other tissue cells. The expression pattern suggests that it may play a role in the regulation of cardiovascular, renal and fertility.
It is found that ACE2 plays an important role in the infection of SARS-Cov and COVID-19. To many researchers, ACE2 has become a hot target due to the recent outbreak of COVID-19.
How does coronavirus infect through ACE2?
The first step of an infection is when coronavirus binds to a functional receptor on the surface of a human cell. For both SARS-Cov and COVID-19, that receptor is ACE2. ACE2 is expressed in alveolar epithelial cells and enterocytes of the small intestine. Both of which are the primary target cells for coronavirus infection.
The surface S protein of the coronavirus binds to ACE2 receptor on the host cell. The affinity of RBD (Receptor Binding Domain) of the S-protein and ACE2 determine the susceptibility of the host. S-protein binds to the claw-like structure of ACE2. The binding creates fusion between the viral and host cell membrane.
The RBD of the S-protein can be engineered to modify its binding affinity with ACE2.
Small molecules or antibodies that can block the binding of S-Protein and ACE2 could be designed as therapeutic agents for the coronavirus infection.
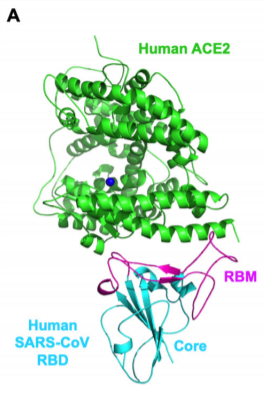
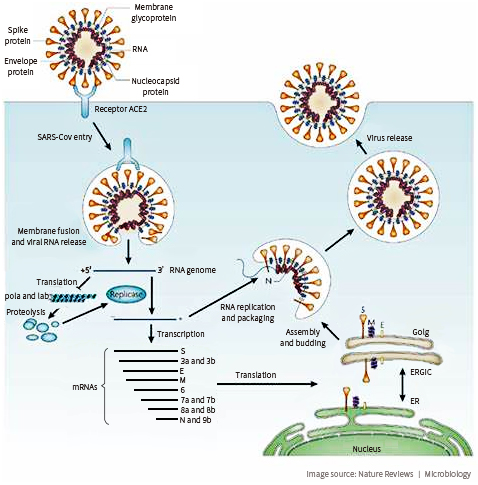
Figure 1 and Figure 2 credit to paper Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS, by Yushun Wan, Jian Shang, Rachel Graham, Ralph S Baric, Fang Li. Journal of Virology, 2020; DOI: 10.1128/JVI.00127-20.
Potential Treatments
Based on the interaction between the S-protein and ACE2 on the cell surface, several potential therapeutic agents have been proposed by researchers to treat coronavirus infection.
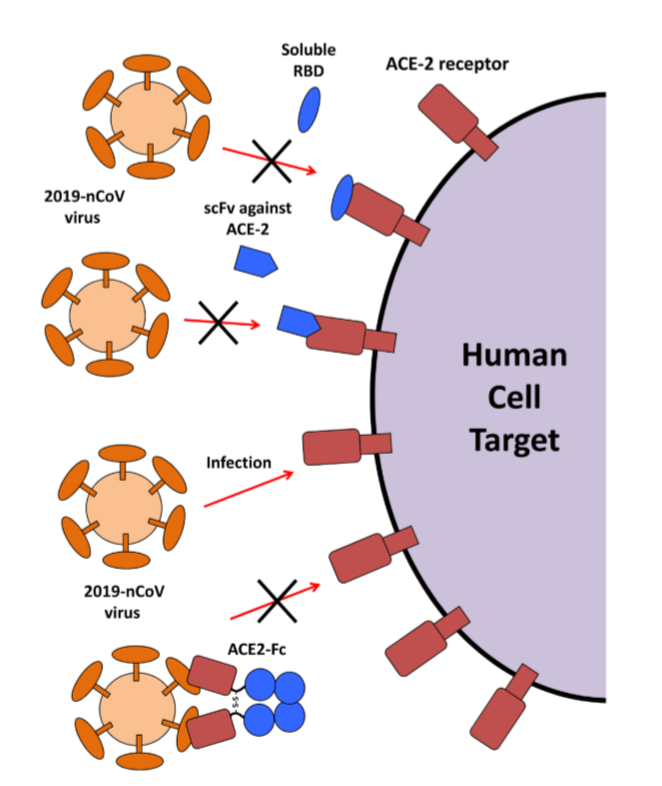
Figure 3 credit to Therapeutic Strategies in an Outbreak Scenario to Treat the Novel Coronavirus Originating in Wuhan, China. [Version 2; peer review: 1 approved], by Kruse RL (2020). https://doi.org/10.12688/f1000research.22211.2
- Use small receptor-binding domain (RBD) from the coronavirus S protein as a blocking agent that binds to the ACE2 receptor.
- Develop an antibody or a single chain antibody fragment (scFv) to bind to ACE2 receptor to prevent coronavirus attachment.
- Use the ACE2 extracellular domain as a bait to bind to the spike protein that directly target coronavirus. This strategy converts soluble ACE2 into an immunoadhesin format fused to an immunoglobulin Fc domain (ACE2-Fc).
Any of the above potential treatments need to be fully tested in animal models and in clinical trials. The potential side effects also need to be investigated.
Biological functions of ACE2
ACE2 is not designed as the receptor of infectious viruses. It also serves many other functions:
ACE2 in Vasodilation
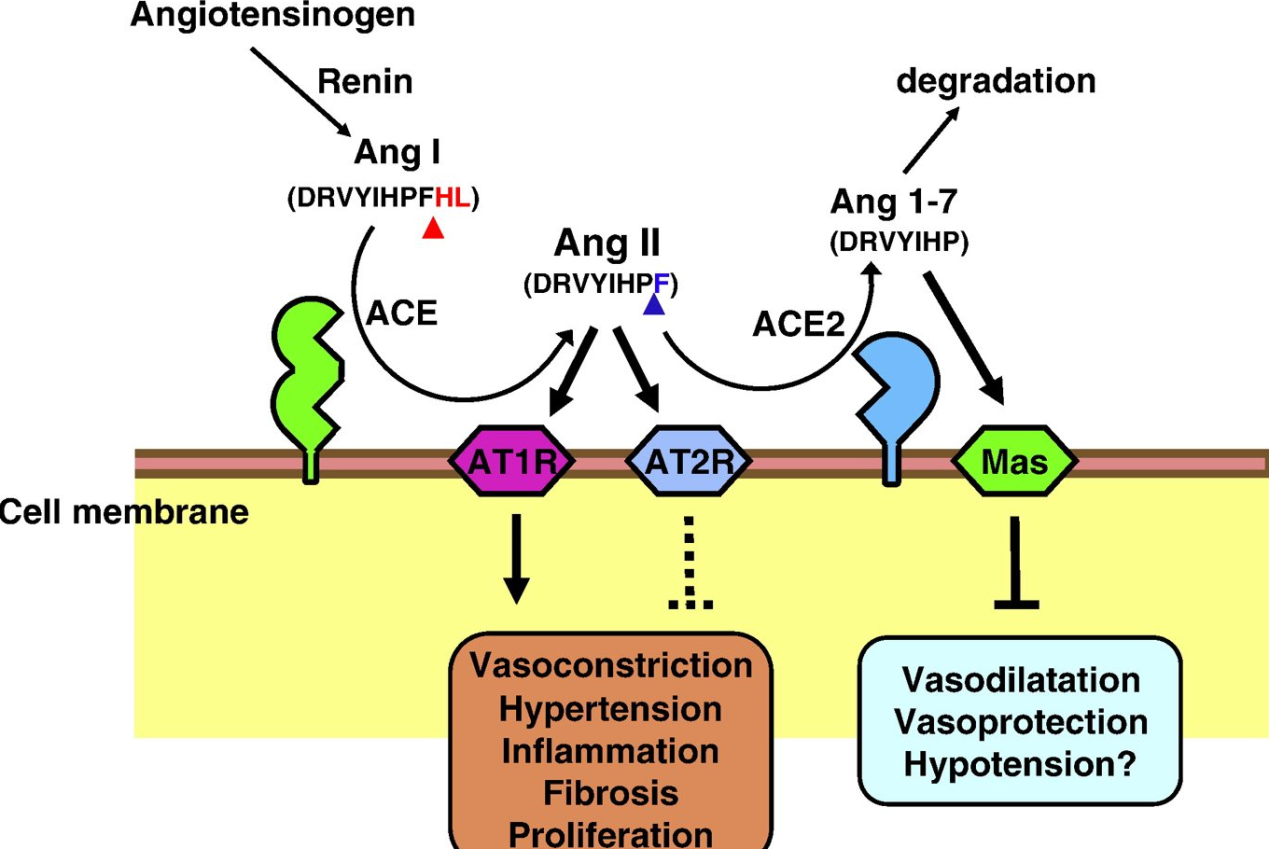
Kuba K et al., (2010). Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 128(1):119-28.
ACE2 is a transporter partner for amino acid
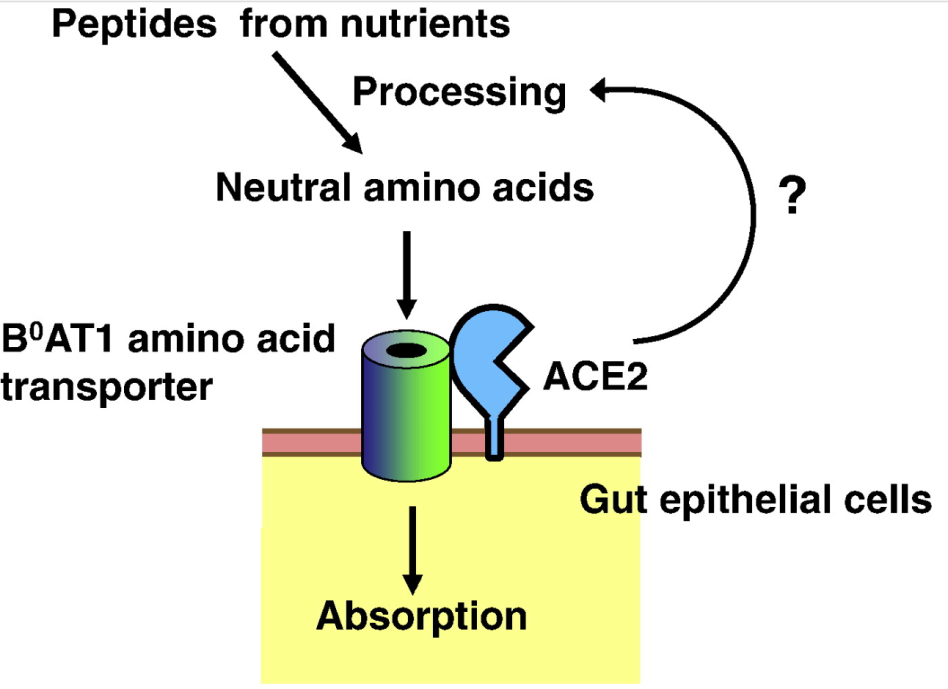
Kuba K et al., (2010). Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 128(1):119-28.
How can we help?
As your gene company, OriGene provides a comprehensive set of tools for ACE2 study including:
- ACE2 Purified Protein
- ACE2 cDNA Clones
- ACE2 Antibodies
- ACE2 RNAi
- ACE2 CRISPR Knock-out Kits
- ACE2 CRISPR Activation Kits
References
- Renhong Yan, Yuanyuan Zhang, Yaning Li, Lu Xia, and Qiang Zhou(2020). Structure of dimeric full-length human ACE2 in complex with B0AT1. doi.org/10.1101/2020.02.17.951848.
- Yushun Wan, Jian Shang, Rachel Graham, Ralph S Baric, Fang Li(2020). Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. Journal of Virology, 2020; DOI: 10.1128/JVI.00127-20.
- Zhu X, Liu Q, Du L, Lu L, Jiang S(2013). Receptor-binding domain as a target for developing SARS vaccines. J Thorac Dis.5 suppl 2:S142-8
- Jiang S. et al (2019). The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat Rev Microbiol. 7:226-36.
- Wu, F. et al (2020). A new coronavirus associated with human respiratory disease in China. Nature https://doi. org/10.1038/s41586-020-2008-3.
- Kuba K et al., (2010). Trilogy of ACE2: A peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. PharmacolTher. 128(1):119-28.
- M. Donoghue, F. Hsieh, E. Baronas, K. Godbout, M. Gosselin, N. Stagliano, M. Donovan, B. Woolf, K. Robison, R. Jeyaseelan, et al (2000). A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9,Circ Res, 87 (2000), pp. E1-E9
- W. Li, M.J. Moore, N. Vasilieva, J. Sui, S.K. Wong, M.A. Berne, M. Somasundaran, J.L. Sullivan, K. Luzuriaga, T.C. Greenough, et al (2003).Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus,Nature, 426 (2003), pp. 450-454


