The Revival of Lentiviral Vectors
Dec 11, 2019


In the early 1990s, researchers set out to harness viruses for gene therapy applications. The idea was to co-opt a virus’ natural ability to target cells, transverse the membrane and deliver genetic cargo for novel gene-based therapies. But like many early-stage technologies, challenges, setbacks and missteps dampened initial enthusiasm.
Scientists spent the next three decades teasing apart the underpinnings of viral biology to create a new generation of vectors. Among these, lentivirus-based vectors, derived from the HIV-1 retrovirus, are of growing interest in gene and cell therapy, particularly in immuno-oncology, due to their ability to infect dividing and nondividing cells, low cytotoxicity rate, capacity for chromosomal integration, and low immune reactivity. In perhaps the best known example, lentivirus-based vectors were instrumental to the development and recent approval of the chimeric antigen receptor (CAR) T cell therapy for a B-cell malignancy1.
Systemic challenges
Current lentiviral vector systems (the third generation) generate virus particles using four plasmids and a producer cell line. The rationale behind four plasmids is to enhance safety, as separating genetic components reduces chances of recombination. The ‘transfer plasmid’ or lentiviral vector contains the transgene of interest, while the ‘envelope plasmid’ contains genes for proteins that appear on the virus surface, the ‘packaging plasmid’ contains core viral components (minus genes for viral replication) and the ‘rev plasmid’ has the viral rev protein. These plasmids are cotransfected into the producer cell line to ultimately generate viral particles ().
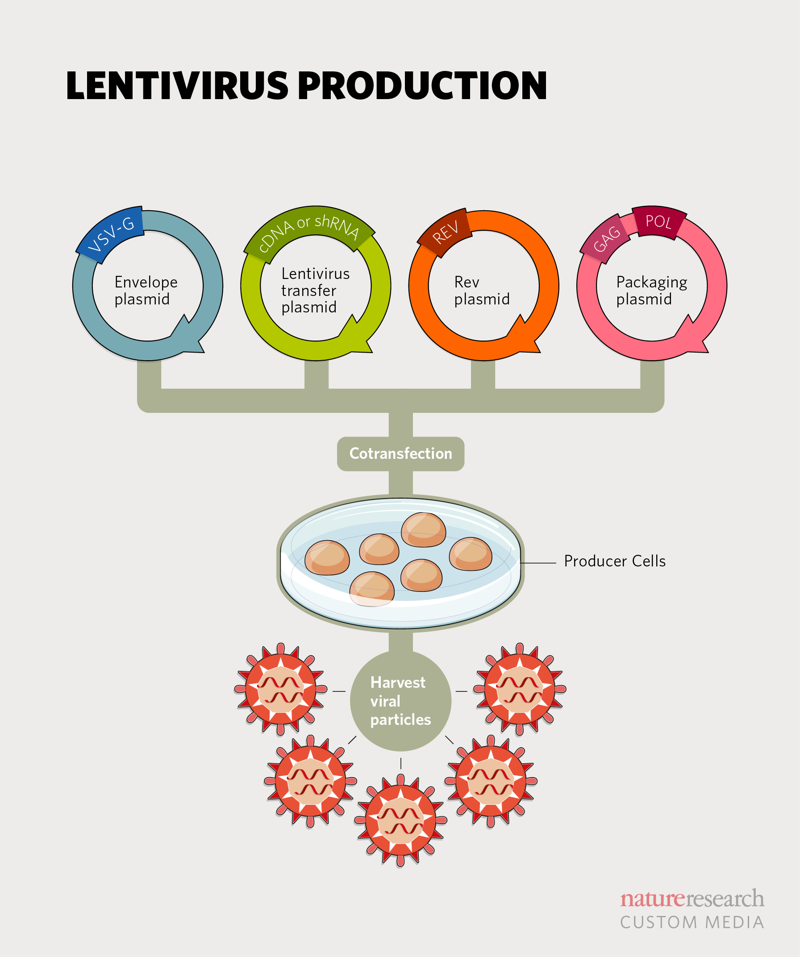
Although lentiviral vector production techniques have matured in recent years with companies such as OriGene Technologies now offering an array of lentiviral vectors and particles to address different needs, systemic delivery of these vectors remains a major hurdle that has stymied gene therapy applications. But there’s movement. In May 2019, a team led by Luigi Naldini and Alessio Cantore, from the San Raffaele Telethon Institute for Gene Therapy, reported a major step towards making systemic delivery safer and more efficient2.
Naldini and Cantore’s goal is to use lentiviral vectors for liver-directed gene therapy of hemophilia, the coagulation disorder. “Since lentiviral vectors can stably integrate into target cells, the approach could enhance treatment prospects in younger patients whose livers are still growing,” explains Cantore. But, early tests using systemic delivery in nonhuman primates showed mild toxicity and low efficacy. Cantore suspected the obstacle was low biodistribution of the lentiviral vector, due to clearance by phagocytes, which was also triggering the innate immune system.
"When we did imaging studies, we saw most macrophages had become vector positive," recalls Cantore. They needed a way to shield lentiviral vectors from attack by phagocytes.
Vector shielding
To protect the viral vector particles, Naldini and Cantore’s team engineered producer cells to overexpress CD47, an integrin associated protein that acts as an avoidance signal for phagocytes. When the researchers examined lentiviral vectors produced in their modified system, they saw increased CD47 staining on the virus surface, and when they tested the vectors in nonhuman primates, they found increased biodistribution and decreased immune response. The results show systemic delivery of lentiviral vectors is feasible, and suggest it might be possible to reduce lentiviral vector doses and still obtain a favorable outcome —further enhancing safety.
Cantore is even more positive as he thinks CD47 overexpression strategies could open doors for gene therapy with other diseases. Naldini and Cantore join the growing list of scientists who have extended the capabilities of lentiviral vectors, helping to create a robust toolkit from which more novel innovations will emerge.
doi: 10.1038/d42473-019-00271-9
- Milone, M., O’Doherty, U., Clinical use of lentiviral vectors. Leukemia 32, 1529–1541 (2018) Google Scholar
- Milani, M., Annoni, A., Moalli, F. et al. Phagocytosis-shielded lentiviral vectors improve liver gene therapy in nonhuman Google Scholar


